Eg Of Chemical Energy
Stored chemical energy is found in food, biomass, petroleum, and natural gas.

Eg of chemical energy. Metabolism is the set of life-sustaining chemical processes that enables organisms transform the chemical energy stored in molecules into energy that can be used for cellular processes. For some examples of potential energy, though, it's harder to see how "position" is involved. Whole or natural foods contain lots of important nutrients.
Rather than using energy from the sun, some will use chemical energy to make their own food. A chemical process that takes place in every cell to release energy. This might be when you you get two objects and they make a fire-maybe an explosion it will create heat energy which would.
Suggest to this list. The signs of internal energy. Nuclear energy is energy stored in interactions between the particles in the atomic nucleus and is studied in nuclear physics.
Chemical energy can be released of absorbed during a chemical reaction. There are two important terms to know in the study of chemical energy. Biology is brought to you with support from the Amgen Foundation.
It is energy that is stored in the bonds between atoms and molecules. Chemical potential energy is the energy stored in the chemical bonds of a substance. Electricity is converted to thermal energy in toasters, radiant energy in light bulbs, and kinetic energy in the electric motors that power washing machines, power tools, fans, and disk drives.
What does EG stand for in Chemical?. Chemical EG abbreviation meaning defined here. Many everyday items store and release chemical energy.
ΔU system = -ΔU surroundings. All autotrophs use non-living material (inorganic sources) to make their own food. When a calorimeter, a device used to measure the heat released by a chemical reaction, the net amount of heat energy that flows through the device is equal to the negative of the total energy change of the system.
Foods, muscles, electrical cells. Donate or volunteer today!. For example, a catalyst could cause a reaction between reactants to happen at a faster rate or at a lower temperature than would be possible without the catalyst.
ΔU isolated system = 0. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The amount of energy and types of nutrients we get is determined by the types of foods we eat.
Chemical energy comes in different forms and may be released during a chemical reaction, usually in the form of heat. This stored chemical energy, or heat content, of the system is known as its enthalpy. Ice particles vibrate slower, but still have energy.
Here we report a fr. Chemical energy is a form of potential energy that will only be observed when it is released in a chemical reaction. Get the top EG abbreviation related to Chemical.
When this occurs, chemical energy can released, an exothermic reaction or chemical energy can be absorbed, an endothermic reaction. The first law of thermodynamics. Rubber balls, springs and elastic bands are like this.
Test prep · MCAT · Chemical processes. Chemical energy may be released during a chemical reaction, often in the form of heat;. The stored energy varies depending on the types such as physical, chemical or even electrical energy.
More rigorous Gibbs free energy / spontaneity relationship. One example of this is the energy stored in gasoline for a car. Such reactions are called exothermic.
Chemical energy from a battery is a potential form of energy, elastic energy in a stretched rubber band is a form of potential energy, but the most commonly referred to form of potential energy in physics is that of gravitational potential energy. Chemical energy is energy that is stored in the bonds between chemical compounds, such as atoms and molecules. It is energy stored in the bonds of chemical compounds, such as sugar and gasoline.
Glucose and oxygen react with each other to produce carbon dioxide and water. Deloitte’s Middle East Oil, Gas & Chemicals professionals work with clients to help maximize business potential across the full spectrum of the industry. From super majors and trading businesses to independents, we deliver services, perspectives and solutions that best suit the business and its people.
This energy is released when the nuclei are combined (fusion) or split. This concept comes from Fermi-Dirac statistics.Electrons are fermions and by the Pauli exclusion principle cannot exist in identical energy states. However, the conventional aqueous electrolytes are not capable of working at low temperature.
Chemical energy in oil is converted to thermal energy to heat our homes. So at absolute zero they pack into the lowest available energy states and build up a "Fermi sea" of electron. Similar to mass balances studied previously, a balance on energy is crucial to solving many problems.
There are other common examples of potential energy. The energy of a. Unhealthy foods do not.
Chemical energy is a form of energy. The stored potential energy often stays in the object until the state of the object changes leading to the release of the energy. The ethylene glycol either gains energy from the source (lake, ocean, water well) or dissipates heat to the sink, depending on whether the system is being used for heating or cooling.
Chemical energy is stored in the bonds of atoms and molecules – it is the energy that holds these particles together. The atomic number is the number of protons an atom has. Autotrophs are any organisms that are capable of producing their own food.
Example:A good example of chemical energy is an electrochemical cell or battery. A ball at the top of a hill stores potential energy until it is allowed to roll to the bottom. In order for a chemical reaction to occur, a substance or substances must change into new substances with different properties.
Chemical energy Chemical Energy is released when bonds form in a chemical reaction, often producing heat as a by-product (exothermic reaction). Energy due to the bonds between particles, sometimes called chemical energy;. When you hold two magnets next to each other, they store potential energy too.
It is characteristic and unique for each element. Energy entering the system is POSITIVE (+), meaning heat. Energy can take several forms — such as heat energy, light energy, electrical energy, and mechanical energy.
Our graduates report being well prepared for careers including food science and engineering, pharmaceuticals, environmental engineering, materials engineering, energy technologies and more. Chemical energy is energy that is stored in chemicals, such as sugar and gasoline. Quenching refers to any process which decreases the fluorescence intensity of a given substance.
This is the currently selected item. Lets discuss various types and examples of potential energy. Potential energy is that energy which is stored in an object.
Atoms of an element that have differing numbers of neutrons (but a constant atomic number) are termed isotopes.Isotopes, shown in Figure 1 and Figure 2, can be used to determine the. Chemical energy is the kind of potential energy "stored" in chemical bonds and is studied in chemistry. Chemical energy is the energy stored within chemicals, which makes its energy inside atoms and molecules.
The ability of food to burn shows that it contains stored energy. Electrical energy is a type of potential energy, or energy stored in an object due to the position of the object. Exothermic reactions release heat and light into their surroundings.
Matter is the other component. Internal Energy Change Equations. Energy is one of two components of the universe.
For most, this is achieved by using light energy, water and carbon dioxide. Where q is heat and w is work. However, it is extremely difficult to measure or even calculate the absolute total of energy in a given chemical system.
Chapter 7 – Energy and Energy Balances The concept of energy conservation as expressed by an energy balance equation is central to chemical engineering calculations. The energy change in a chemical reaction is due to the difference in the amounts of stored chemical energy between the products and the reactants. As chemical energy is stored energy, it is a type of potential energy, which is energy stored in objects due to.
Chemical energy is when there is a chemical reaction which makes energy. Scottish scientist William Rankine first coined the term potential energy in the 19th century. The energy in the chemical bonds of glucose indirectly supplies most living things with a major part of the energy that is necessary for them to carry on their activities.
It is one of the most convenient forms we have for storing energy. Electromagnetic energy is in the form of electric charges, magnetic fields, and photons. Energy is the ability to do work.
A chemical reaction is the process of atoms being rearranged due to a chemical change, resulting in a new substance being produced. Most often, it's considered the energy of chemical bonds, but the term also includes energy stored in the electron arrangement of atoms and ions. Ethylene Glycol Chemistry, Medical, Antifreeze.
A look at a seductive but wrong Gibbs spontaneity proof. Aqueous zinc-based energy storage (ZES) devices are promising candidates for portable and grid-scale applications owing to their intrinsically high safety, low cost, and high theoretical energy density. An oxidizer is a chemical that provides a lot of oxygen to help things release energy.
Galactose, which is rarely found as a simple sugar, is usually combined with other simple sugars in order to form larger molecules. The chemical energy in food is converted by the body into mechanical energy and heat. Chemical Explosions Chemical explosions may be either decomposition or combination reactions.
The energy stored in chemical bonds, such as those between molecules. An exothermic reaction is a form of chemical reaction when heat is produced as a by-product. It's a form of potential energy that you won't observe until a reaction occurs.
Our mission is to provide a free, world-class education to anyone, anywhere. Experiences like these set Bucknell chemical engineering graduates apart, and nearly all get jobs or enroll in graduate school within six months. As a consequence, quenching is often heavily dependent on pressure and temperature.Molecular oxygen, iodide ions and acrylamide are common chemical quenchers.
Reactions that require an input of heat to proceed may store some of that energy as chemical energy in newly formed bonds. Interesting Facts about Potential Energy. In the case of electrical energy, the object is the charged particle, and the.
Developing the science and technology needed to produce biofuels and renewable energy technologies such as solar cells and advanced batteries for plug-in hybrid electric vehicles (PHEVs) has become crucial as the world moves to employ alternatives to petroleum-based fuels. There are different types of chemical energy, such as electrochemical energy and chemiluminescence. The various chemicals that make up gasoline contain a large amount of chemical potential energy that is released when the gasoline is burned in a controlled way in the engine of the car.
Chemical Energy Chemical energyresults from chemical reactionsbetween atoms or molecules. Kinetic energy and potential energy. Plants convert radiant energy from the sun into chemical energy via photosynthesis.
Decomposition reactions occur in materials such as trinitrotoluene (TNT) and nitroglycerine. But two general types of energy are especially important to chemists:. Interestingly, when chemical energy is released, the substance from which the energy came is often changed into an entirely new substance.
EG Chemistry Co., Ltd Company Description EG Chemistry leads new millenium in the fields of Excision Chemical Industry and Environmental Industry in the World.We provide complete corrosion protection in variety of forms to suit your steel products. Some objects can change shape reversibly. Fermi Level "Fermi level" is the term used to describe the top of the collection of electron energy levels at absolute zero temperature.
Nuclear energy is stored in the nucleus of atoms. An isolated system cannot exchange heat or work with its surroundings making the change in internal energy equal to zero. Chemical energy, Energy stored in the bonds of chemical compounds.
Chemical energy is one of the various forms energy can take, including kinetic energy, mechanical energy, and thermal energy. In either case, the reaction is exothermic and the energy released by the reaction is partially converted to work. Chemical - Chemical potential energy is the energy stored up in substances due to their chemical bonds.
Energy liberated by a chemical reaction or absorbed in the formation of a chemical compound. Pure ethylene glycol has a specific heat capacity about one half that of water. All living organisms need energy to grow and reproduce, maintain their structures, and respond to their environments.
Biology is brought to you with support from the. Biofuels and Energy Technologies. Khan Academy is a 501(c)(3) nonprofit organization.
The release of that energy does two things. This is energy that is stored due to an object's position. Chemical energy is the energy of chemical bonds and is also stored in atoms and ions.
These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisional quenching. A chemical catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that catalyst.

Radiant To Chemical Egee 401 Energy In A Changing World
C03 Apogee Net Contentplayer Coursetype Kids Utilityid Srpnet Id

The First Law Of Thermodynamics Introduction To Chemistry
Eg Of Chemical Energy のギャラリー

Chemical Reaction Definition Equations Examples Types Britannica
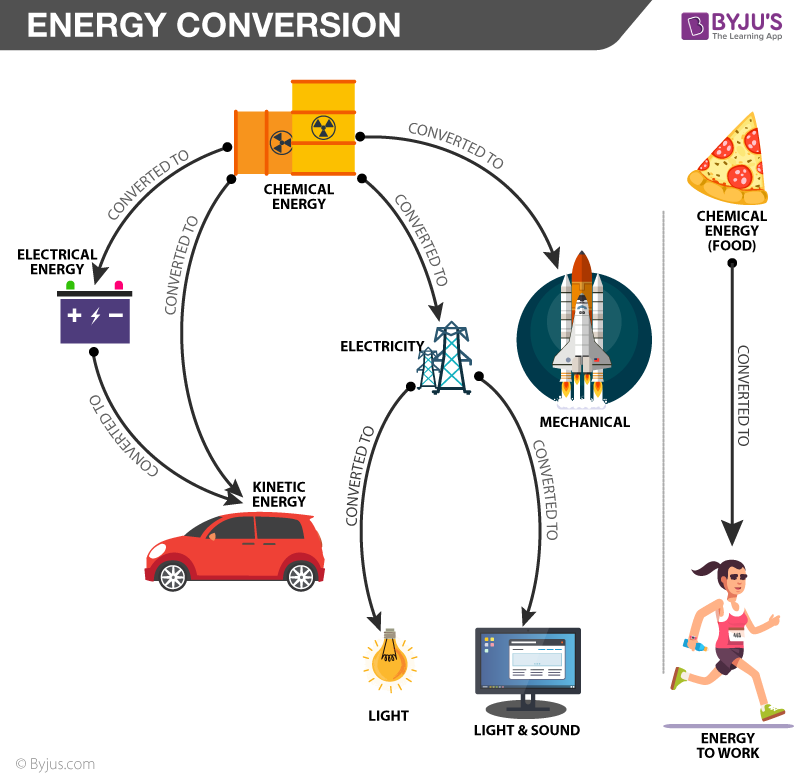
Energy Conversion Law Of Energy Conversion With Examples
Energy Types Of Energy Young People S Trust For The Environment

Thunderbolt Kids
/main-energy-forms-and-examples-609254-v3-5b562a0cc9e77c0037514831.png)
10 Types Of Energy And Examples
Www Flippedoutscience Com Uploads 2 7 8 2 Energy Transformations Notes Pdf

Potential Energy Definition Examples Facts Britannica

Chemical Reactions Introduction Video Khan Academy
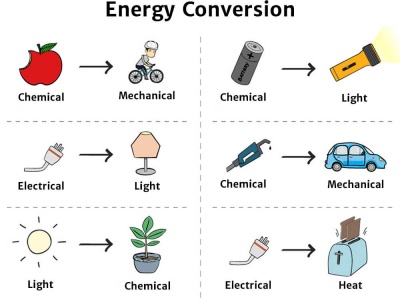
Energy Conversion Knowledge Bank Solar Schools
Elasto Chemical Energy Of G Assuming That The Mechanically Stronger A Download Scientific Diagram

Examples Of Energy Transformation In Daily Life Teachoo
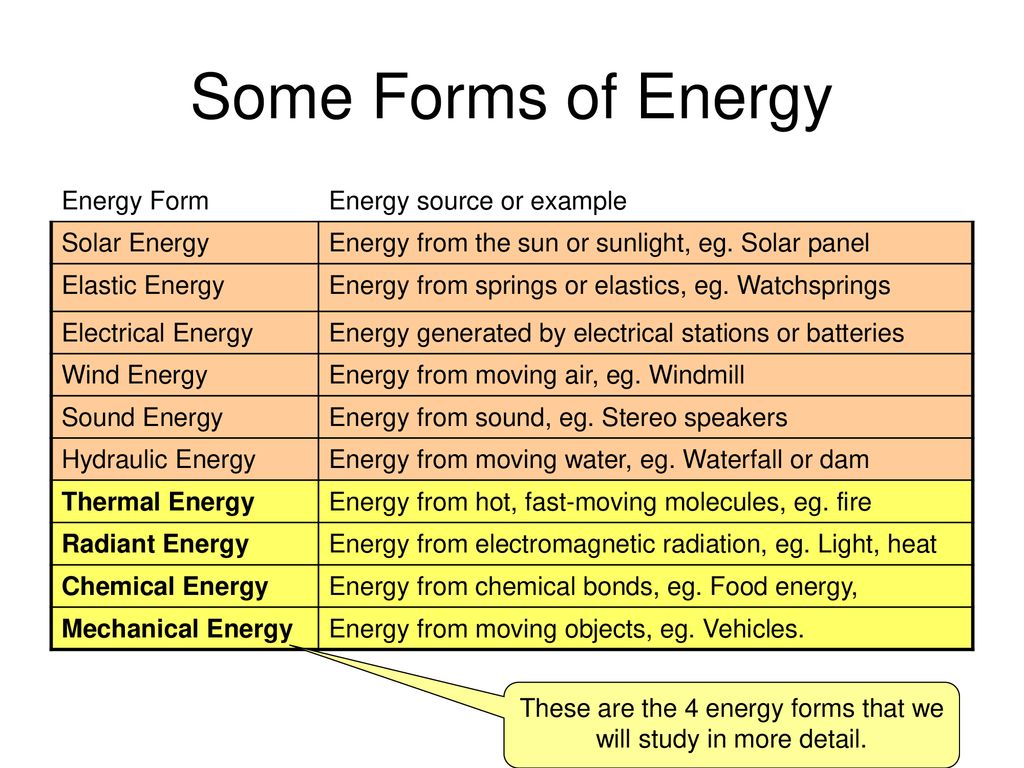
The Human Organism And The Power Of Energy Ppt Download

Green Chemistry Examples American Chemical Society

Examples Of Chemical Energy

Conservation Of Energy In Chemical Reactions Ck 12 Foundation

Examples Of Chemical Reactions In Everyday Life
Q Tbn 3aand9gctix7a3cwxjn Lxxcmuts15xob11nyfjdvwooc Nztl54prsu79 Usqp Cau

Chemical Energy Knowledge Bank Solar Schools

Types Of Energy Knowledge Bank Solar Schools

Types Of Energy Types Of Energy Electrical Energy Light Energy Sound Energy Thermal Energy Chemical Energy Nuclear Energy Mechanical Energy Each Can Ppt Download

Advanced Energy Storage Devices Basic Principles Analytical Methods And Rational Materials Design Liu 18 Advanced Science Wiley Online Library
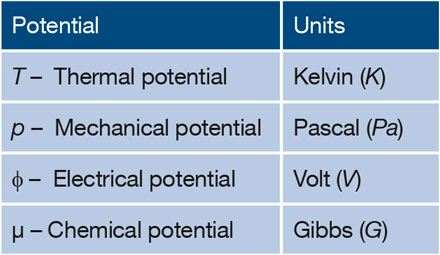
Chemical Potential And Gibbs Free Energy
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)
12 Examples Of Chemical Energy
Solved Question 42 The Amount Of Chemical Energy Passed F Chegg Com

13 Examples Of Kinetic Energy In Everyday Life Studiousguy

Types Of Energy Article Khan Academy

Energy Sources In An Eaf E G Electrical Energy And Chemical Energy Download Scientific Diagram

Chemistry For Renewable Energy Master S Programme In Chemistry 21 Uppsala University Sweden
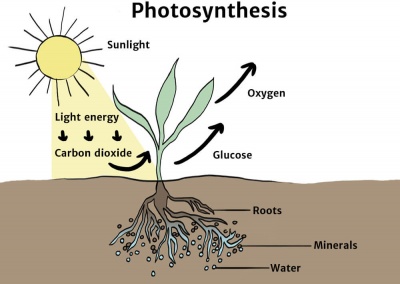
Chemical Energy Knowledge Bank Solar Schools

Energy Energy Benchmark Sc B The Student Recognizes Various Forms Of Energy E G Heat Light And Electricity Also Assesses B 1 2 3 B 1 2 4 Ppt Download
Photosynthesis Converts Solar Energy Into Chemical Energy Plants Asknature

1 Name The 9 Types Of Energy Giving Examples Ppt Download

Energy Conversion All You Need To Know And More
Chemical Energy Storage

Oxidative Phosphorylation

Difference Between Physical And Chemical Change With Examples
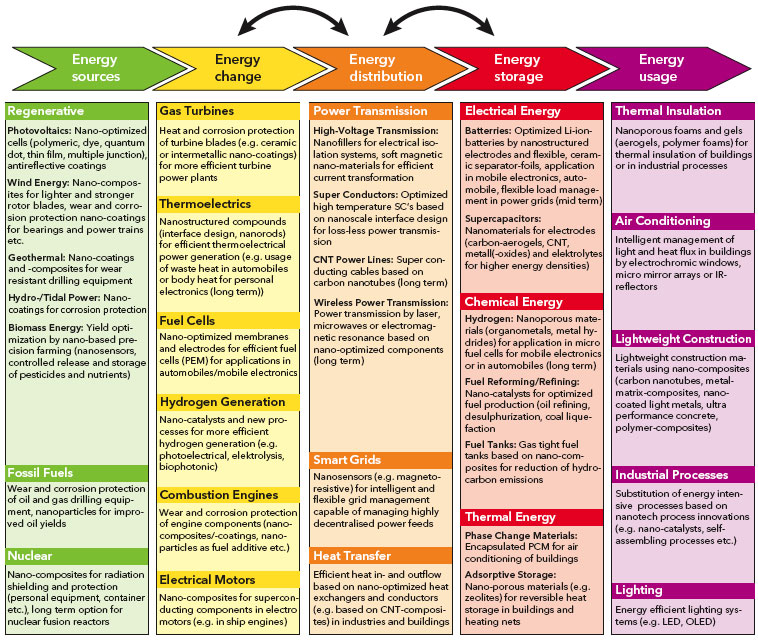
Nanotechnology In Energy

What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com

Chemical To Electrical Egee 401 Energy In A Changing World
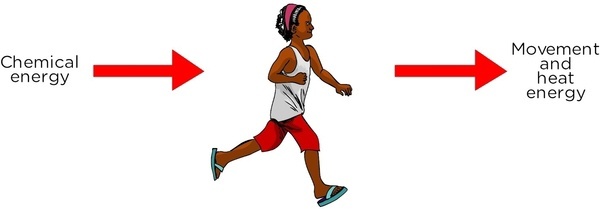
Thunderbolt Kids

Block3 Html
3

What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com

What Are Some Examples Of Potential Energy

Energy Changes In Chemical Reactions Introduction To Chemistry

Readings Energy

Energy Wikipedia
Energy Types Of Energy Young People S Trust For The Environment

Energy Transformation Wikipedia
Www Flippedoutscience Com Uploads 2 7 8 2 Energy Transformations Notes Pdf

Study Guide For Science Standard 6 2 New
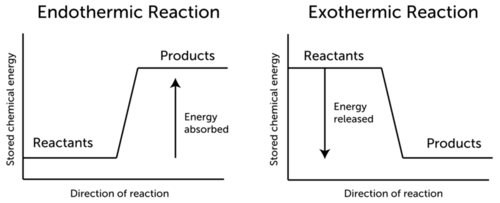
Conservation Of Energy In Chemical Reactions Ck 12 Foundation
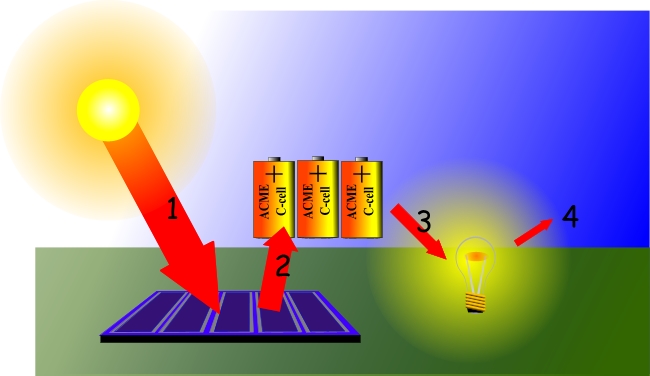
Energy Types Of Energy Young People S Trust For The Environment

Energyflow

Chemical Energy Knowledge Bank Solar Schools

Q Tbn 3aand9gctsbkvq2fvyzzo Isdf545aqvg2pcessqcnkw Usqp Cau

What Is Chemical Energy Definition Examples Applications With Videos
Www Flippedoutscience Com Uploads 2 7 8 2 Energy Transformations Notes Pdf

Exothermic Reaction Wikipedia

Why Is Mass Conserved In Chemical Reactions Science Questions With Surprising Answers
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)
12 Examples Of Chemical Energy
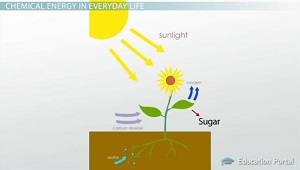
Energy From The Sun Green Plants As Primary Food Producers Tomatosphere First The Seed Foundation

Examples Of Potential Energy

Chemical Energy Knowledge Bank Solar Schools

Igcse Chemistry Energy Changes And Calorimetry Youtube

Thermal Energy Lab Welcome

Types Of Energy Different Forms Of Energy Physics Youtube
What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com

Energy And Metabolism Boundless Biology

Examples Of Chemical Change Definition Examples With Videos

A The Scaled Ground State Energy Eg Ef B The Chemical Potential Download Scientific Diagram

Chemical Changes

Energy Transformation Definition Types Examples Video Lesson Transcript Study Com
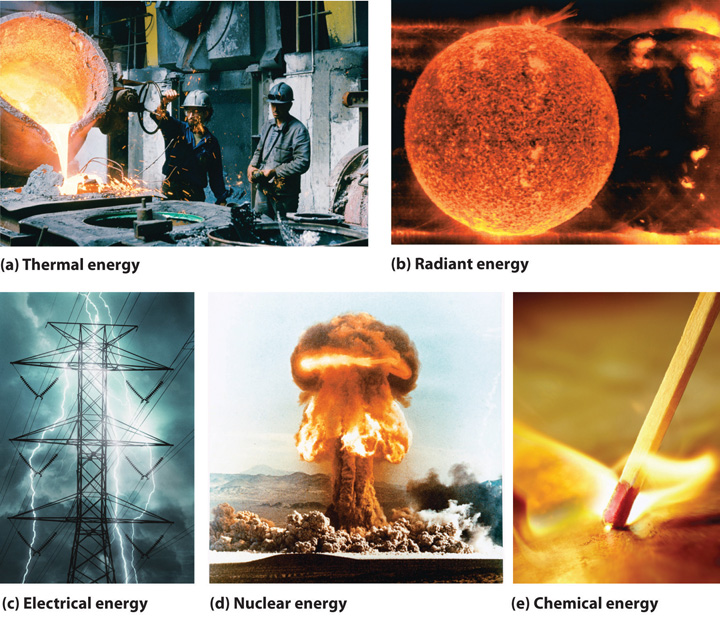
Energy And Work

What Is Thermal Energy Definition Examples Video Lesson Transcript Study Com

Study Guide For Science Standard 6 2 New
Energy Types Of Energy Young People S Trust For The Environment

1st Period 6th Grade Unit 4 Flashcards Quizlet

Biodotedu

Nuclear Energy Definition Sources Uses Facts Britannica
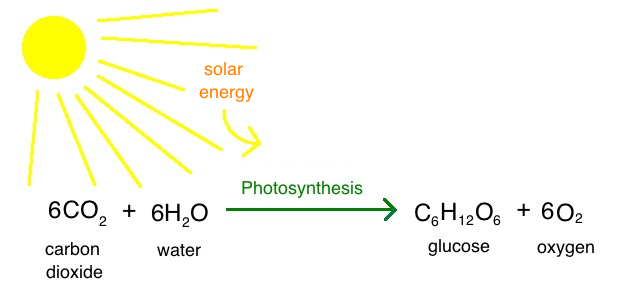
Intro To Photosynthesis Article Khan Academy

Chemical Energy An Overview Sciencedirect Topics
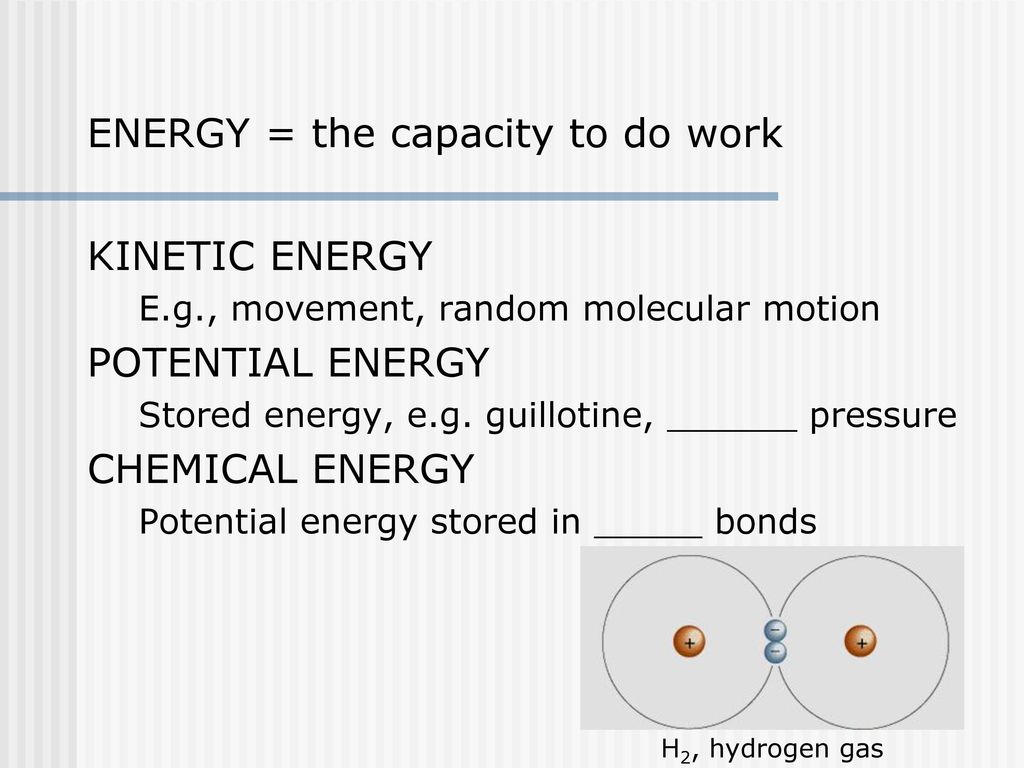
The Harvest And Storage Of Chemical Energy Ppt Download
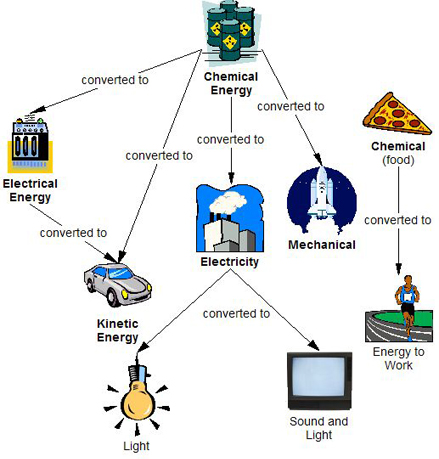
Energy Conversion Egee 102 Energy Conservation And Environmental Protection

Solved Using Atp To Provide Energy For Biological Process Chegg Com

Solved Kinetics Broadly Speaking The Factors That Influe Chegg Com
Q Tbn 3aand9gcsqjcksjgfatid8dexgjp4njdhbv7zbqvscccglvdblxa Jdktk Usqp Cau

Work Work Work Is Said To Be Done When The Point Of Application Of A Force Moves And It Is Measured Using The Product Of Force And The Distance Moved Ppt
1
Ei Lehigh Edu Learners Energy Readings Energy Basics Pdf

Examples Of Mechanical Energy At Home And In Daily Life

1st Period 6th Grade Unit 4 Flashcards Quizlet
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)
12 Examples Of Chemical Energy

Energy

Classification Of Organisms Based On Their Primary Source Of Energy Download Scientific Diagram
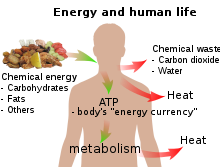
Energy Wikipedia

Structural Formula Definition Examples Video Lesson Transcript Study Com

Chemical Energy Knowledge Bank Solar Schools

Forms Of Energy Transformations Of Energy And Their Real World Applications

Bond Enthalpy And Enthalpy Of Reaction Article Khan Academy
/TC_608334-chemical-change-examples-5aabebea31283400371a753e.png)
Chemical Change Examples In Chemistry



